NMR Spectroscopic Analysis of Tetradecyl Trimethyl Ammonium Chloride
Jan 17, 2024Nuclear magnetic resonance spectrometer is a high-precision instrument with very high detection sensitivity. Nuclear magnetic resonance spectroscopy is widely used in structural determination of drugs and organic chemicals. The use of nuclear magnetic resonance spectroscopy can easily and accurately analyze the purity of macromolecules or salt organic substances. The sensitivity is not comparable to conventional analytical methods such as gas chromatography and titration analysis. Ahsuperchem uses nuclear magnetic resonance technology to determine the purity of Tetradecyl trimethyl ammonium chloride, especially to detect the content of free amines and amine salts (detection limit at least<0.1%).
1. Prepare samples
Take 10-15 mg of the sample and place it in a nuclear magnetic tube. Add 0.5 mL of deuterated chloroform (CDCl3) and shake to dissolve. If it cannot be completely dissolved, heat the nuclear magnetic tube appropriately.
2. Tests
Measure the chemical shift using tetramethylsilicon as the internal standard on a nuclear magnetic resonance instrument. Generally, nuclear magnetic resonance imaging machines have specialized. The person in charge is responsible, and the laboratory technician can operate according to conventional parameters.
3. Material structure spectrum analysis
According to the standard database spectrum and our own sampling analysis, the nuclear magnetic resonance hydrogen spectrum data of pure Tetradecyl trimethyl ammonium chloride is 1H-NMR (CDCl3, ppm) δ: 0.88 (t, 3H, J 7.2 Hz), 1.26-1.35 (d, 22H), 1.74 (s, 2H), 3.45 (s, 9H), 3.54 (m, 2H), briefly explained as follows: Tetradecyl trimethyl ammonium chloride (molecular formula CH3(CH2)11CH2CH2N(CH3)3Cl) has a total of five signal peaks in the nuclear magnetic resonance response, indicating the presence of five types of hydrogen. The chemical shift represents the type of hydrogen, and the peak area represents the number of hydrogen. a represents the methyl hydrogen at the end of the fourteen carbon chain, with a chemical shift of 0.88 ppm, consisting of three hydrogen atoms; b represents the methylene hydrogen on the middle 11 carbon atoms of the fourteen carbon chain, with a chemical shift of 1.26-1.35 ppm, totaling 22 hydrogen atoms; c represents the methylene hydrogen on the 13th carbon atom of the fourteen carbon chain, with a chemical shift of 1.74 ppm, consisting of two hydrogen atoms; d represents the methylene hydrogen on the carbon atom adjacent to the nitrogen atom on the fourteen carbon chain, with a chemical shift of 3.54 ppm, consisting of two hydrogen atoms; e represents the methyl hydrogen on the nitrogen atom, with a chemical shift of 3.45ppm, consisting of 9 hydrogen atoms.
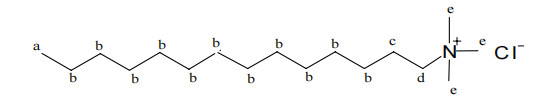
Figure 1 shows the nuclear magnetic resonance hydrogen spectrum of Tetradecyl trimethyl ammonium chloride produced by us on December 16, 2023. After comparison, it can be found that it is completely consistent with the standard spectrum, indicating that the structure of our product is consistent with the standard sample.
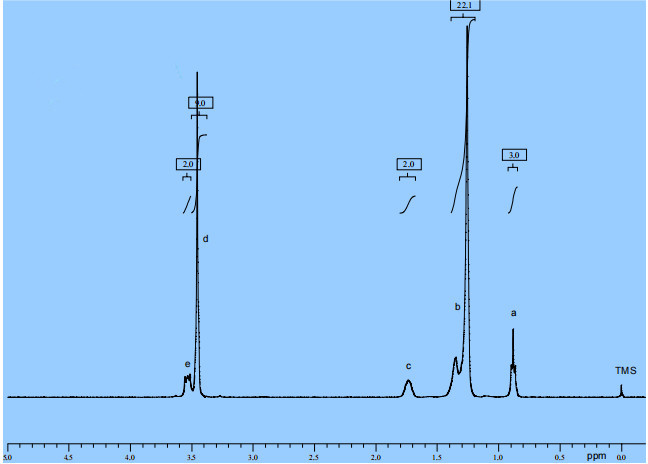
Figure 1