Direct Extraction of Tungsten from Simulated Tungsten Ore Soda Leachate Using TOMAC
Aug 10, 2021In the metallurgical process of tungsten, tungsten minerals are mostly decomposed using alkaline methods (soda or sodium hydroxide) to obtain leachate containing a large amount of free NaOH or Na2CO3, as well as small amounts of impurities such as P, As, and Si. The main processes for extracting tungsten from the alkaline leachate to produce ammonium paratungstate (APT) are ion exchange and solvent extraction. Due to the high water consumption and wastewater discharge, as well as the high cost of wastewater treatment associated with ion exchange, solvent extraction has become widely accepted.
Principle of solvent extraction: The process of extracting tungsten and preparing ammonium tungstate solution using quaternary ammonium salts includes steps such as extraction, stripping, and regeneration. The extractant is quaternary ammonium carbonate, the stripping agent is a mixture of ammonium bicarbonate and ammonium carbonate, and the regeneration agent is a mixture of sodium hydroxide and sodium carbonate. The main reaction equations for the process are as follows:
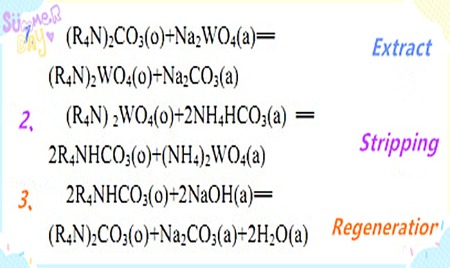
Extraction Experiment: Trioctylmethylammonium Chloride was chosen as the extractant, iso-octanol as the polarizing agent, and sulfonated kerosene as the diluent. The organic phase composition was 50% (volume fraction) sulfonated kerosene. TOMAC was an industrial-grade product with a purity of 90%. Iso-octanol was chemically pure. Before extraction, the CL-type quaternary ammonium salts in the organic phase were converted to CO2-3 type. The feed solution was prepared using chemically pure reagents to simulate the composition of industrial tungsten ore soda leachate. The stripping agent and regenerating agent were also prepared using chemically pure reagents. The extraction and stripping experiments were conducted in a separatory funnel on a temperature-controlled oscillator.
Extraction Conclusion: The concentration of Na2CO3in the feed solution had little effect on the extraction rate of WO3, but it had a significant impact on the phase separation performance during the extraction process. The extraction system using TOMAC as the extractant was able to effectively extract tungsten from high-concentration Na2CO3 systems. When the organic phase composition was 50% TOMAC + 30% iso-octanol + 20% sulfonated kerosene, the saturation extraction capacity for tungsten reached 91.49 g/L WO3.