Sep 21, 2022
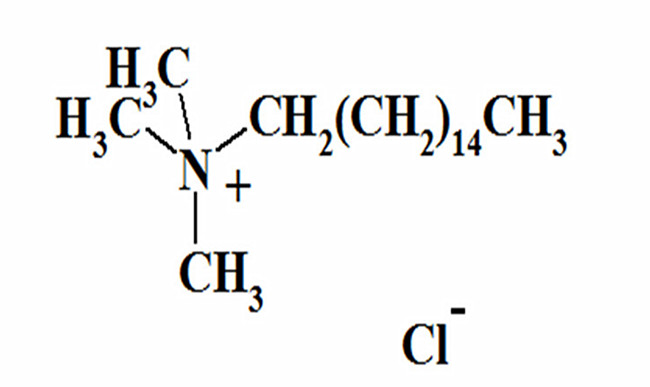
Quaternary ammonium salt fungicides are sprayed on crops in the field, and the factors that affect the effectiveness of fungicides in disease prevention in the field are not limited to the three aspects of pesticides, environment, and crops. However, the application technology of fungicides requires higher requirements than that of insecticides and herbicides. Especially, it is necessary to fully understand the occurrence and development laws of diseases, as the occurrence and development of diseases are not as clear as pests and weeds.
Two points to pay attention to when spraying crops in the field:
Firstly, it is the type and concentration of the medication. The choice of medication type depends on the type of disease, so it is necessary to make a correct diagnosis of the disease type before applying the appropriate medication to the case. For example, rice blast can be treated with rice blast, rice blast, tricyclazole, etc. For wheat powdery mildew and rust, triadimenol, triadimefon, etc., and for peanut leaf spot disease, methoxytropizine, etc.
Secondly, it should be noted that if the same disease occurs on different crops, sometimes the same pesticide cannot be used. For example, Bordeaux liquid can prevent and control downy mildew, but it is easy to cause pesticide damage to Chinese cabbage, so it is not suitable to prevent and control downy mildew in Chinese cabbage. After selecting the type of pesticide, it is necessary to choose the appropriate application concentration based on the crop type and growth period, the type and dosage form of fungicides, and environmental conditions.